Tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF) are prodrugs of tenofovir (TFV) that form a major component of most prophylactic and therapeutic regimens for the treatment of HIV-1 globally. However, TDF is rapidly cleaved in plasma and in the liver by esterases to generate systemic TFV, which accumulates in the kidney, causing nephrotoxicity over the course of chronic treatment. The release of TFV in plasma has also been linked to bone mineral density depletion. Although TFV plasma exposure is dramatically reduced with TAF relative to TDF, a large percentage of TAF is extracted by the liver due to cleavage by carboxylesterase 1. As a result, only small fraction of TAF is available to access target HIV infected cells.
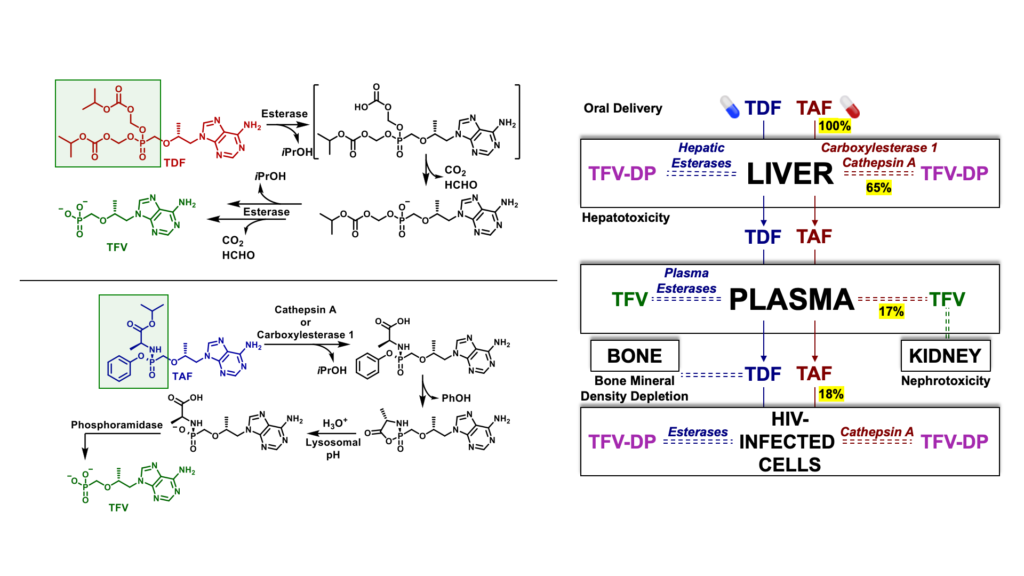
Another prodrug of TFV, tenofovir exalidex (TXL) was derived from lysoglycerophospholipids, such as phosphatidylcholine. Although TXL was designed to rely primarily on cleavage by phospholipase C to release TFV intracellularly, like TAF, TXL is readily metabolized in the liver by CYP450 omega-hydroxylases. This undesired metabolism by the liver not only compromises the amount of prodrug available to access HIV infected cells, but also, due to chronic use, increases the potential for organ-specific toxicities which can negatively impact adherence to treatment.
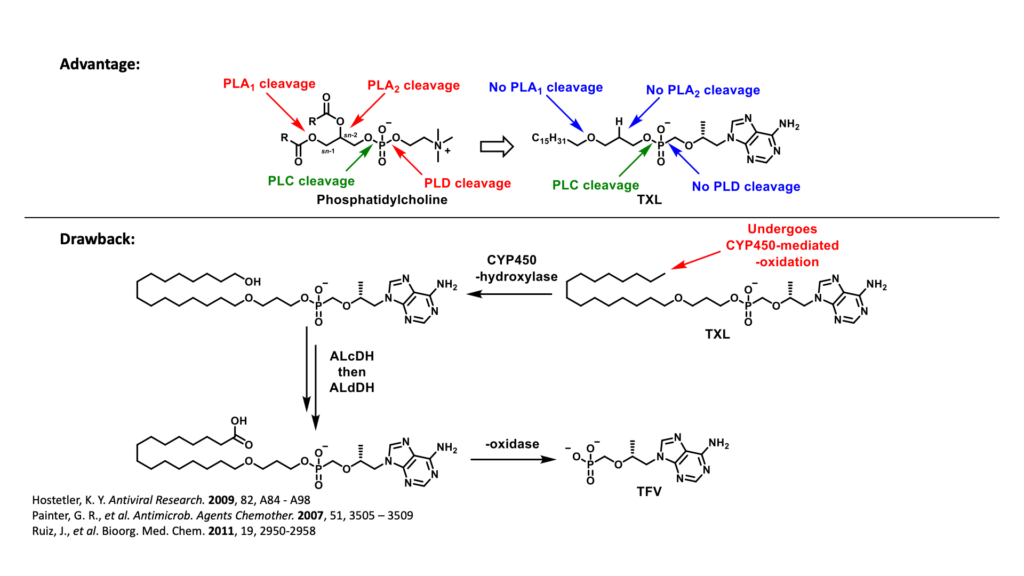
To overcome the limitations exhibited by TXL, TAF and TDF, our current research efforts are focused on the design and synthesis of a series of TXL lipid analogues able to mitigate undesired CYP450-mediated ω-oxidation. One of these efforts is directed to replacing the labile terminal methyl group with various structural motifs with diminished sensitivity to hepatic metabolism. It was hypothesized that the introduction of these metabolically stable motifs would, in turn, minimize TFV-associated toxicities of the bone, kidney and liver. In addition, these prodrugs have the potential to improve the efficacy of TFV-based regimens by directing a larger fraction of the administered dose to HIV-infected cells.
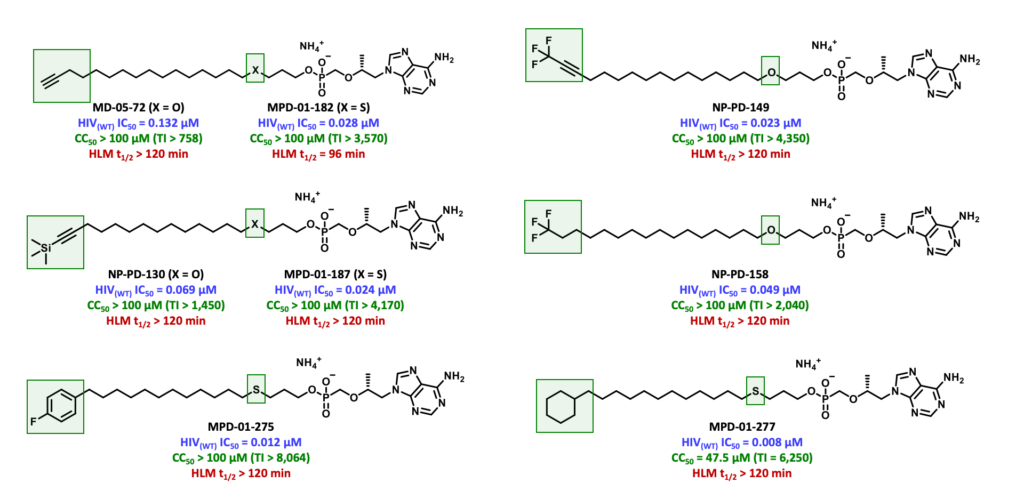
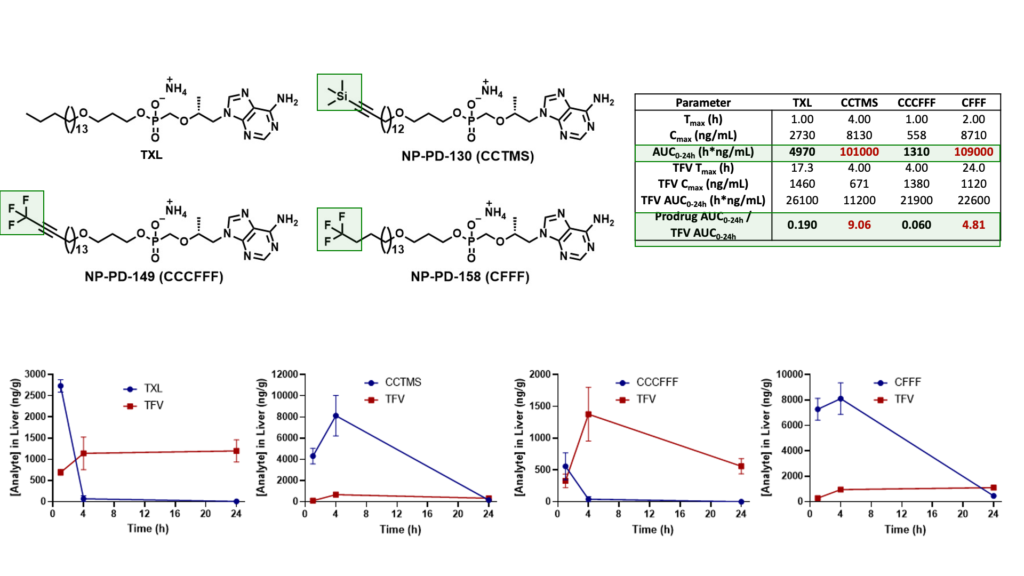
We have since identified various potent TXL lipid analogues that have demonstrated improved HLM stability in vitro relative to TXL. Furthermore, terminally substituted TXL lipid analogues exhibited enhanced systemic exposure levels in vivo when compared to TXL and relatively superior stability in mouse plasma and liver.
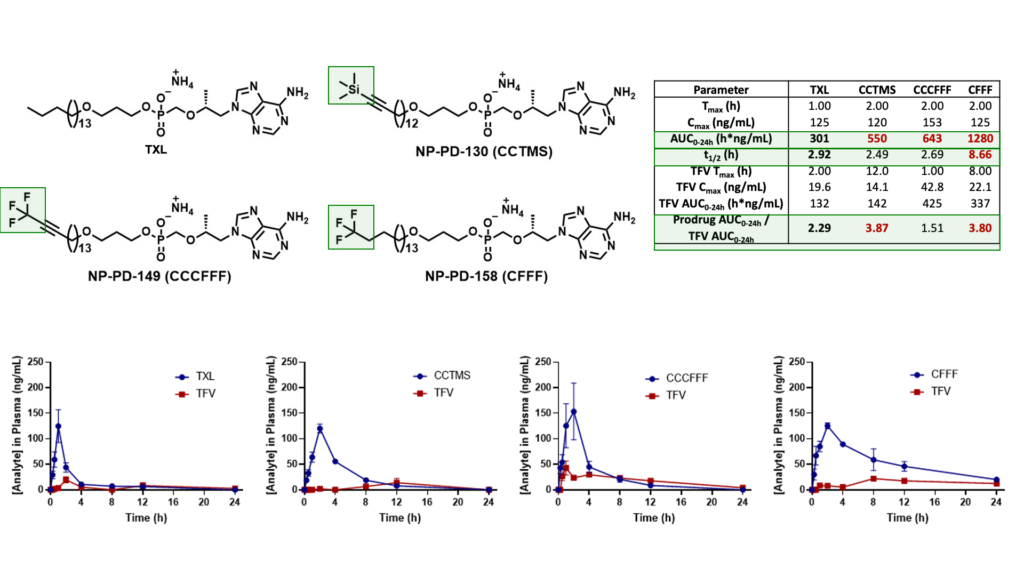
Mouse Plasma PK Results
Male C57BL/6 mice (n = 3 per time point) were administered a single oral dose (10 mg/kg) of TFV prodrug using 90:10 olive oil: EtOH as a vehicle. Levels of prodrug and TFV were quantified using LC-MS/MS.
Mouse Liver PK Results
Male C57BL/6 mice (n = 3 per time point) were administered a single oral dose (10 mg/kg) of TFV prodrug using 90:10 olive oil: EtOH as a vehicle. Levels of prodrug and TFV were quantified using LC-MS/MS.