The NMDA receptor belongs to the ionotropic glutamate receptor family and is responsible for mediating excitatory neurotransmission in the central nervous system. It is a heterotetramer, made up of two glycine-binding GluN1 subunits with 8 splice variants and two glutamate-binding GluN2 subunits with 4 subtypes (2A to D), which render the receptor with different expression patterns in the brain. This ligand-gated ion channel is permeable to Ca2+, Na+ and K+ ions and has a voltage dependent Mg2+ block.
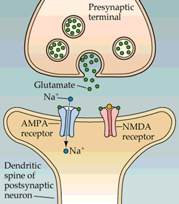
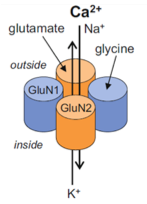
The NMDA receptor is critical for nearly every function of the brain and is involved in learning, memory, brain development and synaptic plasticity. NMDA receptor dysfunction has been implicated in numerous neurological disorders, including Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, schizophrenia and depression.
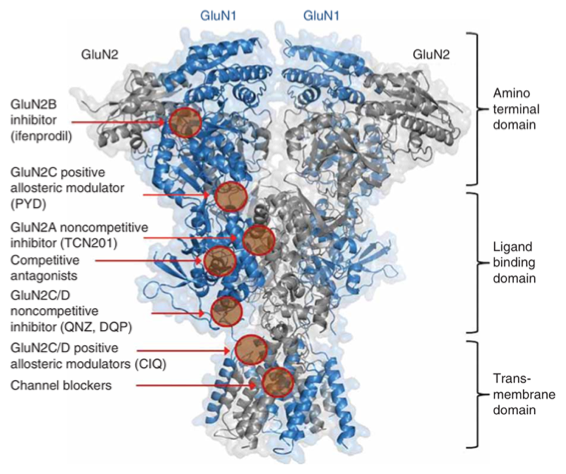
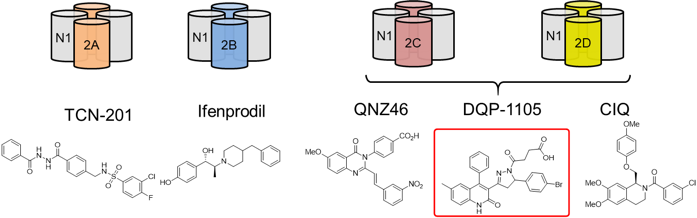
Over a decade ago, the Liotta and Traynelis Research Groups started their drug discovery endeavor in the NMDA receptor (NMDAR) field. Multiple series of subunit-selective NMDAR modulators were identified by a high-throughput screening study. Subsequent medicinal chemistry campaigns were carried out in the Liotta Research Group to explore the structure-activity relationship in each series of NMDAR modulators. These medicinal chemistry campaigns led to numerous publications and multiple clinical candidates. The most notable one is NP-10679, a GluN2B-selective, pH-dependent NMDAR modulator. Following a successful Phase I clinical trial, NP-10679 was recently granted Orphan Drug Designation by the FDA for the treatment of subarachnoid hemorrhage.
