Building and Creating.
Dr. Liotta puts his words in to action.
Dr. Liotta is dedicated to innovating new approaches to drug development in academia. He aims to overcome many intrinsic (and often unforeseen) barriers associated with academic drug development, such as inadequate infrastructure, insufficient expertise and lack of translational funding.
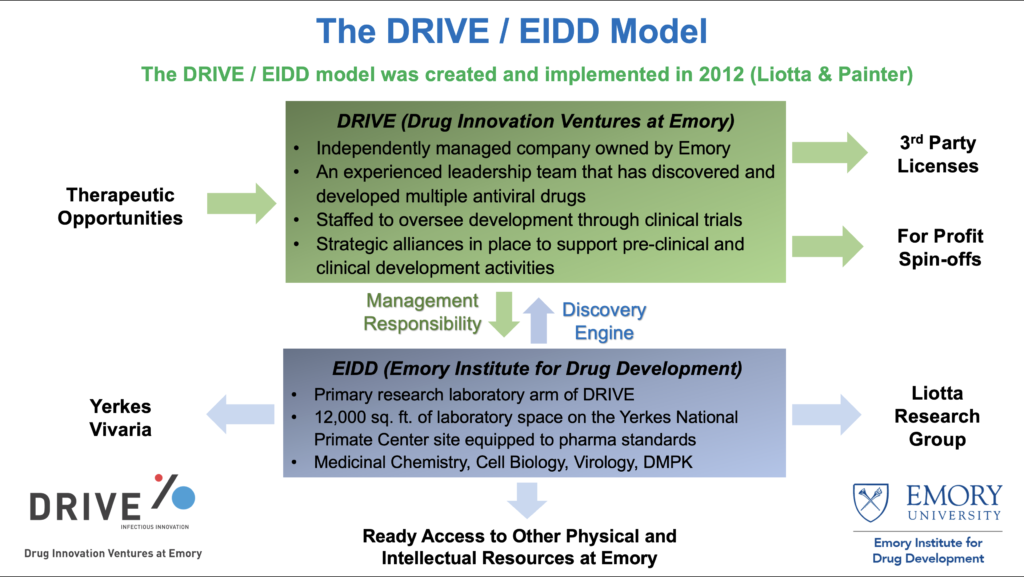
He and fellow drug developer, Dr. George Painter, set up an innovative model a decade ago to bolster academic drug development infrastructure and provide critical resources in the developmental continuum for new therapeutics. In 2009-12, Dr. Liotta funded the creation of a pair of complementary entities at Emory University – Emory Institute for Drug Development (EIDD) and Drug Innovation Ventures at Emory (DRIVE). These two entities form a strategic alliance to advance drug development at Emory, one of the first of its kind in the nation. The EIDD, equipped with state-of-the-art facilities and highly trained research personnel, serves as an engine for early-stage research and development. DRIVE, staffed by an experienced leadership team having critical expertise in business development, intellectual property law, and pre-clinical and clinical research, acts as a commercialization partner to advance drug candidates to subsequent value inflection points. Revenues generated from out-licensing the pre-clinical and clinical assets are channeled back to EIDD and DRIVE to fund new drug discovery campaigns.
Without shareholders or investors, EIDD and DRIVE strive to address the most critical unmet medical needs rather than pursue markets with the highest profit margins like their commercial sector counterparts. For example, EIDD and DRIVE focus on developing therapeutics for viral diseases of global concern. Many of these diseases had not been commercially viable targets for large pharmaceutical companies or privately held biotech companies.
Over the years, the DRIVE model has generated successes across multiple research fronts. For instance, the EIDD won several multi-million-dollar government contracts in antiviral drug discovery. The EIDD and DRIVE also developed many promising antiviral agents, some of which were subsequently out-licensed to commercialization partners. One example is ATI-2173 (formerly EIDD-2173), a novel, orally available antiviral agent that can potentially provide a cure for hepatitis B. ATI-2173 is out-licensed to Antios Therapeutics, which recently raised $96 million in Series B financing to fund its Phase 2 clinical trials. Aside from antiviral agents, the EIDD also worked on various other therapeutic areas. For example, it discovered NTS-104 (formerly EIDD-1723), a novel neurosteroid prodrug for treating stroke and traumatic brain injuries. NTS-104 is licensed to NeuroTrauma Sciences and is poised to enter clinical trials in the last quarter of 2022.
The EIDD and DRIVE’s efforts to develop therapeutics for commercially neglected diseases unexpectedly provided a critical advancement against the COVID-19 pandemic. Molnupiravir (formerly EIDD-2801), a broad-spectrum antiviral agent initially developed by EIDD and DRIVE for addressing multiple single-stranded RNA virus infections, provided a pivotal solution for COVID-19 treatment. Now licensed to Merck, this drug is currently one of only two oral medications granted Emergency Use Authorization by the FDA for treating SARS-CoV-2 infections. To date, Merck has supplied molnupiravir to more than 25 countries, including the U.S., which purchased 3.1 million treatment courses ($2.2 billion). The New York Times reported this exciting story. It commented, “in the race for a COVID-19 pill, a little lab [at Emory] plays a big role.”
Dr. Liotta documented the DRIVE model in an article published in ACS Medicinal Chemistry Letters. This model will serve as a practical guide for restructuring academic drug development among other universities and research institutions. Without resting on the success of the DRIVE model, Dr. Liotta is currently working on a new initiative to strategically expand the therapeutic scope of the EIDD/DRIVE and implement operational capacity to make this platform available to a broader scientific community at Emory University.
Dr. Liotta catalyzed more than 30 license agreements between Emory University and various commercial partners, including at least 25 exclusive license agreements and seven non-exclusive license agreements. Through these license deals, many therapeutic agents developed in Dr. Liotta’s laboratory were advanced to the clinic and beyond by the commercialization partners.
As a serial entrepreneur, Dr. Liotta co-founded more than 10 biotech companies, including Altesa Biosciences, AgriThera, Que Oncology, NeurOp, Pharmasset, Altiris Therapeutics, Triangle Pharmaceuticals, Metastatix, FOB Synthesis and iThemba Pharmaceuticals. These business fostered economic growth, created numerous jobs in the biotechnology sector, and brought multiple drugs to the marketplace. One prominent example Pharmasset, which developed sofosbuvir (Sovaldi), a blockbuster, curative hepatitis C medicine. Pharmasset was acquired by Gilead Sciences for 11.2 billion in 2012. Another notable example is Triangle Pharmaceuticals, which advanced emtricitabine through its Phase 3 clinical trials before being acquired by Gilead Sciences for $525.2 million in 2003.
Dr. Liotta is currently serving or previously served on the Scientific Advisory Boards of more than a dozen biotech companies and venture capital firms. His knowledge of medicinal chemistry and experience in drug discovery and development have provided critical support to these companies’ technological advancement and commercial growth.
